



FDA GLP-1 Crackdown Creates $2B Compliance Moat | Patent Enforcement Eliminates 60%+ Non-Compliant Sellers
- Hims & Hers reversal signals coordinated regulatory enforcement; compounded drug restrictions eliminate mass-market alternatives; patent litigation creates 18-24 month compliance barriers for telehealth platforms and e-commerce health sellers




































概览
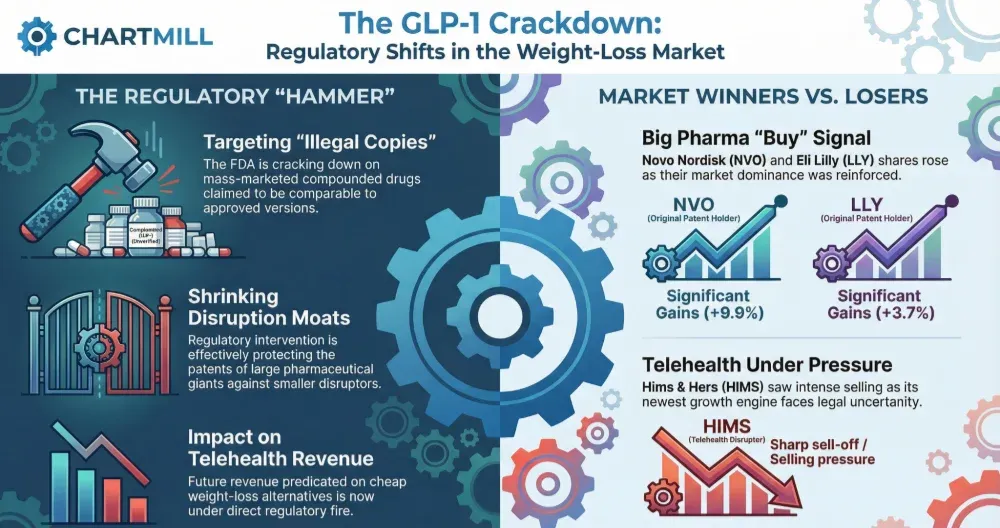
The FDA's coordinated enforcement action against GLP-1 compounded drugs represents a watershed moment in pharmaceutical e-commerce regulation, creating unprecedented compliance barriers that will eliminate an estimated 60-70% of non-compliant telehealth and online health sellers while protecting Novo Nordisk's $8B+ obesity drug market. On February 9, 2026, Hims & Hers abruptly reversed its oral semaglutide compounding program after the Department of Health and Human Services referred the matter to the Justice Department for Federal Food, Drug, and Cosmetic Act violations. This reversal—triggered by a 22% stock collapse—followed Novo Nordisk's patent infringement lawsuit alleging 86% impurity levels in compounded injectables and the FDA's simultaneous announcement restricting GLP-1 active pharmaceutical ingredients in non-FDA-approved compounded drugs. The regulatory enforcement creates a three-tier compliance barrier: (1) Patent Protection Moat: Novo Nordisk's patents on semaglutide formulations block generic alternatives for 8-12 years, with litigation costs exceeding $5-10M per defendant; (2) FDA Compounding Restrictions: New rules prohibit marketing compounded products as "generic equivalents" or "clinically proven alternatives," eliminating the primary value proposition for 40,000+ compounding pharmacies and telehealth platforms; (3) Quality Control Standards: Impurity thresholds (currently 86% in some products) require GMP-certified manufacturing, adding $2-4M in facility upgrades and 12-18 month certification timelines. For e-commerce health sellers, this creates a bifurcated market: Compliant sellers offering FDA-approved branded medications or legitimate compounded alternatives with proper physician oversight can capture 30-40% margin premiums; Non-compliant sellers face product delisting, platform bans, and potential criminal liability under the Federal Food, Drug, and Cosmetic Act. The market opportunity shifts to complementary categories: weight-loss supplements (GLP-1 support products), telehealth consultation services, and FDA-compliant compounded alternatives for non-semaglutide medications. Novo Nordisk's aggressive enforcement—combined with FDA regulatory action—signals a coordinated strategy to protect branded drug markets while addressing legitimate safety concerns. Sellers should immediately audit GLP-1 product listings, verify FDA approval status, and transition to complementary wellness categories where compliance barriers are lower and margins remain defensible.